Bohr Model Atomic Number Thorium Periodic Table Symbol Mercury
The Bohr model is a neat but quite imperfect depiction of the inner workings of an atom before things got too muddled up by quantum principles.. It may orbit at the distance of Mercury, then.
Mercury (Hg) AMERICAN ELEMENTS
9.4: The Bohr Model - Atoms with Orbits is shared under a CK-12 license and was authored, remixed, and/or curated by Marisa Alviar-Agnew & Henry Agnew. LICENSED UNDER. Bohr's model suggests that each atom has a set of unchangeable energy levels, and electrons in the electron cloud of that atom must be in one of those energy levels.
PPT Mercury (Hg) PowerPoint Presentation, free download ID6592903
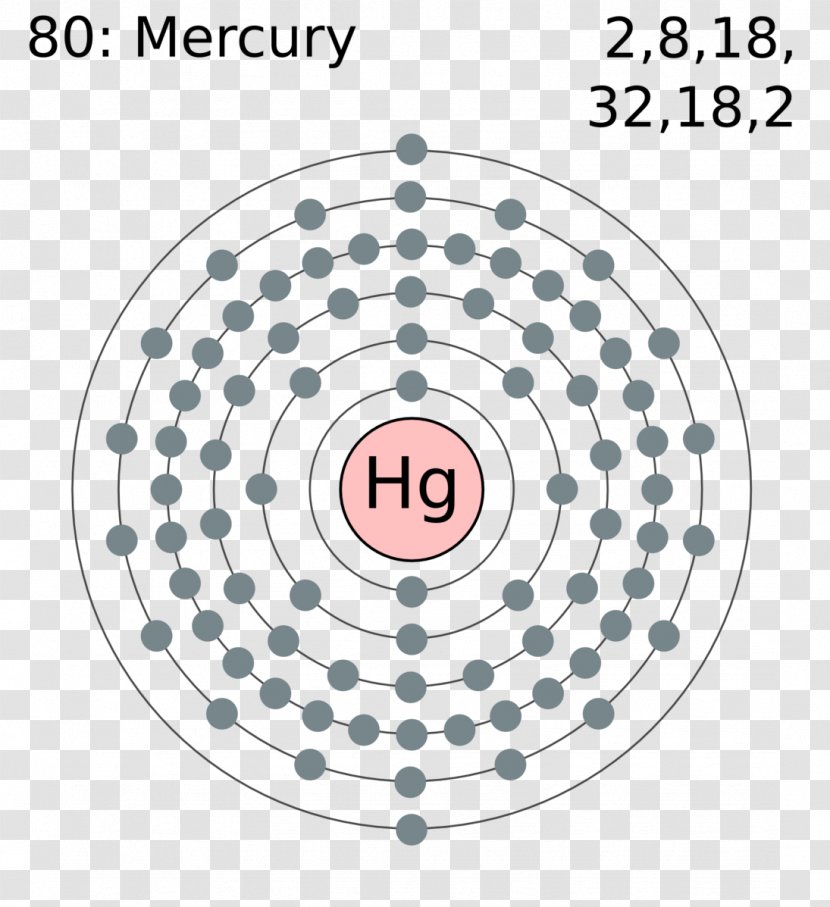
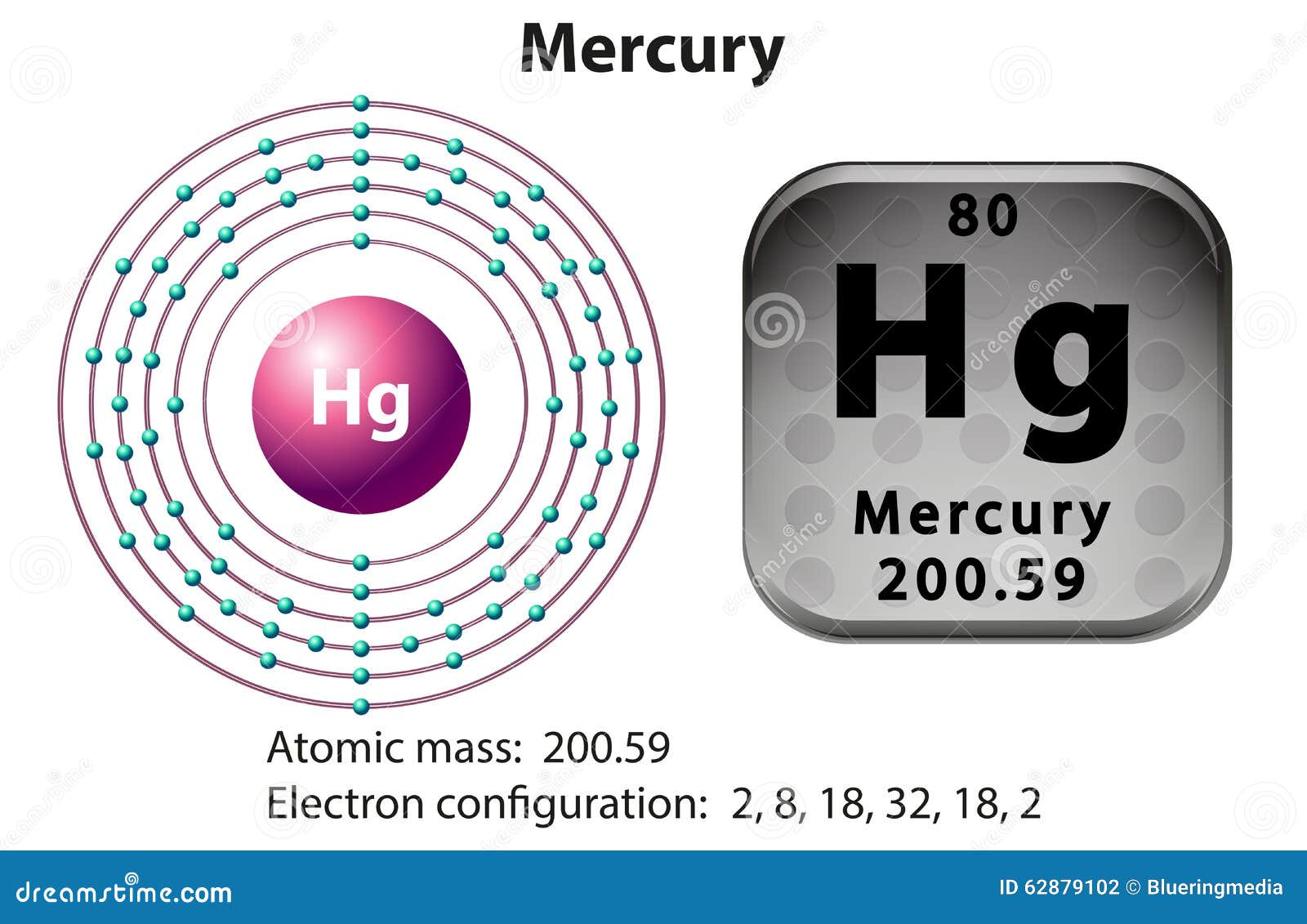
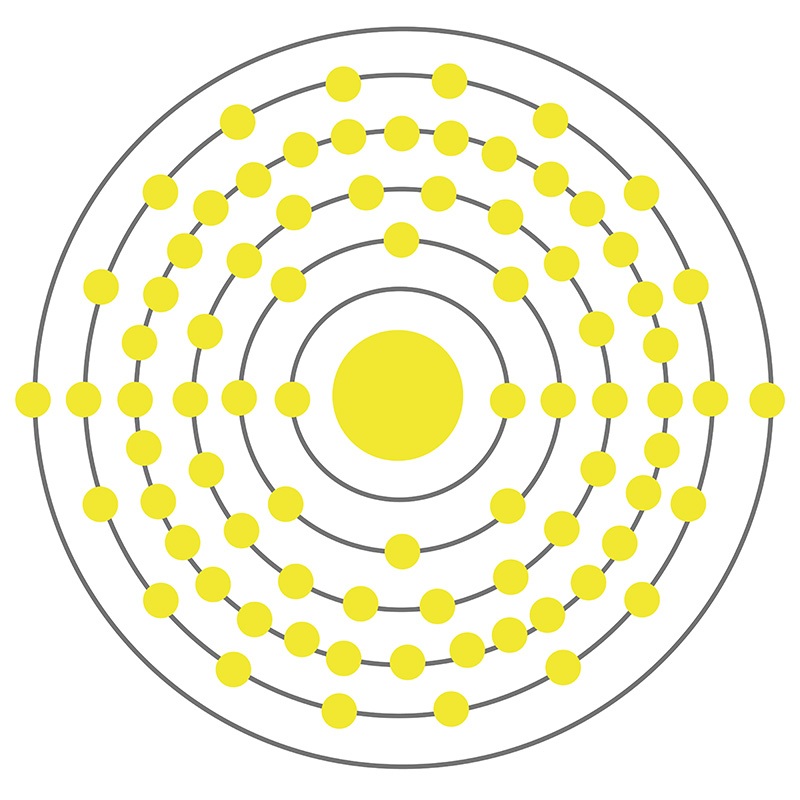
The Bohr Model of Mercury (Hg) has a nucleus that contains 121 neutrons and 80 protons. This nucleus is surrounded by six electron shells. The first shell of the Bohr diagram of Mercury has 2 electrons, the 2nd shell has 8, the 3rd shell has 18, the 4th has 32, the 5th has 18, and the 6th shell has 2 electrons. Also check -

What is the electron configuration of mercury
Bohr model, description of the structure of atoms, especially that of hydrogen, proposed (1913) by the Danish physicist Niels Bohr.The Bohr model of the atom, a radical departure from earlier, classical descriptions, was the first that incorporated quantum theory and was the predecessor of wholly quantum-mechanical models. The Bohr model and all of its successors describe the properties of.
Hg Mercury Element Information Facts, Properties, Trends, Uses and
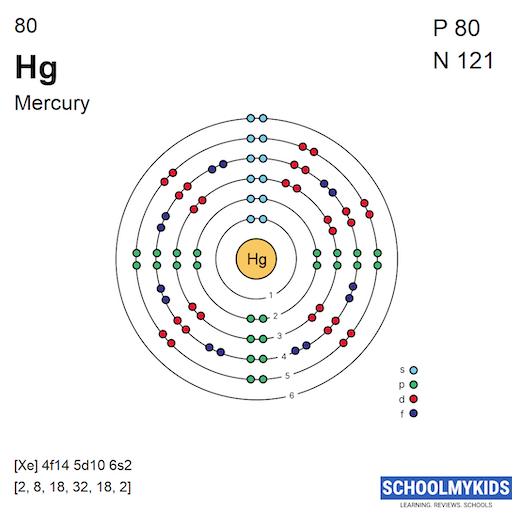
Methods We can write the electron configuration of mercury using four different methods: #1 Using aufbau principle #2 Using periodic table #3 From its Bohr model #4 From its orbital diagram Let's break down each method in detail. Using aufbau principle First, find electrons of mercury atom Periodic table

Mercury (element)
Figure \(\PageIndex{2}\): The Bohr Model of the Hydrogen Atom (a). Similarly, the blue and yellow colors of certain street lights are caused, respectively, by mercury and sodium discharges. In all these cases, an electrical discharge excites neutral atoms to a higher energy state, and light is emitted when the atoms decay to the ground state.

Mercury Atom Vector Graphic Download Free Vector Art, Stock Graphics
In this way, it can be explained why mercury, for example, emits a specific energy spectrum to which specific wavelengths (colors) in the light spectrum belong. The figure below shows the emitted spectrum of a mercury-vapor lamp.. Although the Bohr model of the atom is a further development of Rutherford's atomic model, it also contains.
Symbol And Electron Diagram For Mercury Stock Vector Image 62879102
which is identical to the Rydberg equation in which R ∞ = k h c. R ∞ = k h c. When Bohr calculated his theoretical value for the Rydberg constant, R ∞, R ∞, and compared it with the experimentally accepted value, he got excellent agreement. Since the Rydberg constant was one of the most precisely measured constants at that time, this level of agreement was astonishing and meant that.

Bohr Model Representation Mercury Atom Number Stock Vector (Royalty
The Bohr model represents the particle nature of electrons. So, it's easy to see that the atom above contains two electrons. As we'll discuss later in the article, atomic electrons exist at specific energy levels. The Bohr model represents these energy levels as rings.

Related image Electron configuration, Mercury, Element
The Bohr Model. In 1913, a Danish physicist, Niels Bohr (1885-1962; Nobel Prize in Physics, 1922), proposed a theoretical model for the hydrogen atom that explained its emission spectrum.. neon; and (c) mercury. The strongest lines in the hydrogen spectrum are in the far UV Lyman series starting at 124 nm and below. The strongest lines in.

Mercury Atom Project Atom project, Projects, Crafts for kids
Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. Bohr's model calculated the following energies for an electron in the shell, n : E ( n) = − 1 n 2 ⋅ 13.6 eV
Bohr electron configuration of mercury >> mall man gat box bionicle
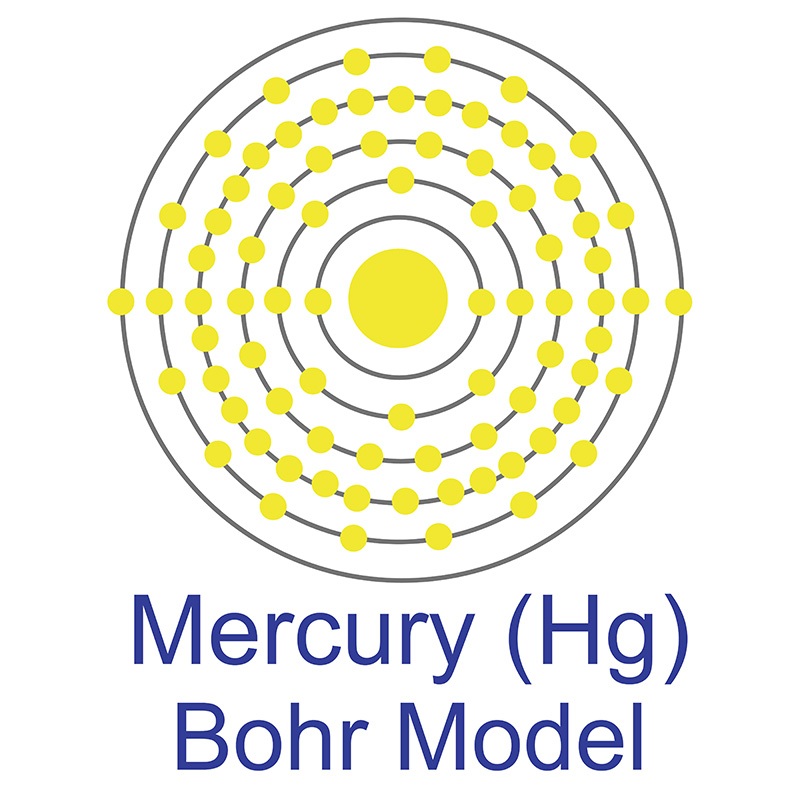
The Bohr Model has an atom with a positively-charged nucleus surrounded by negatively-charged electrons that have circular, planetary-like orbits. Today, we know that the Bohr Model has some inaccuracies, but it's still used because of its simple approach to atomic theory.

Complete Electron Configuration for Mercury (Hg)
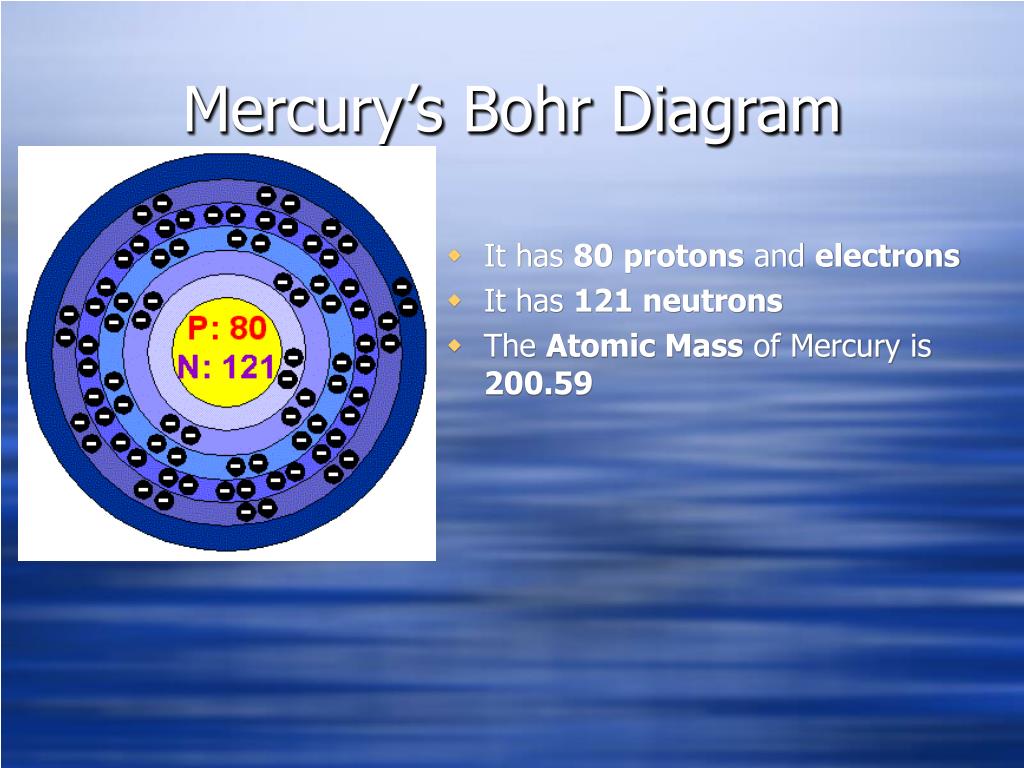
The Bohr model of mercury contains a nucleus having 80 protons and 120 neutrons in the center, and around this nucleus, there are six electron shells containing 80 electrons. Contents Steps #1 Write protons, neutrons, and electrons of mercury atom #2 Draw nucleus of mercury atom #3 Draw 1st electron shell #4 Draw 2nd electron shell

IMovie Element Project by Kat O'Neill
Figure 7.3.2 7.3. 2: The emission spectra of sodium and mercury. Sodium and mercury spectra. Many street lights use bulbs that contain sodium or mercury vapor. Due to the very different emission spectra of these elements, they emit light of different colors. The lines in the sodium lamp are broadened by collisions.
Mercury (Hg) AMERICAN ELEMENTS
Bohr Model of all Elements (Diagrams + Chart) March 23, 2023 by Jay Bohr model of all Elements is mentioned in the chart below. Free Gift for you: Interactive Periodic Table Let me tell you how this Interactive Periodic Table will help you in your studies. 1).
Mercury Bohr Model Clipart (3494266) PinClipart
From the Bohr model, it can be found that the number of orbits or shells in mercury is 6. Hence, as mercury has 6 orbits, it lies in period 6 of the Periodic table. Why is Mercury in d-block? Before knowing this reason, first of all I want to ask you a simple question. How can you determine the blocks-wise position of elements?
