Solved Hydrogen Energy Level DiagramThe orbitals of
Energy Level Diagrams. Prior to 1922, atomic emission was used to qualitatively identify elements, but was too imprecise for quantitative analysis. Lester Strock developed the use of internal standards ( see our page on internal standards as well) to compensate for the sample-to-sample and time-dependent variations in signal generation from a.
iGCSE CHEMISTRY REVISION HELP Acids & Energetics
An energy level diagram, or energy band diagram, is a graphical representation of the allowed energy states of a system. The most common type of energy level diagram is the molecular orbital energy level diagram. This type of diagram shows the energies of the orbitals in a molecule.

Energy Diagram — Overview & Parts Expii
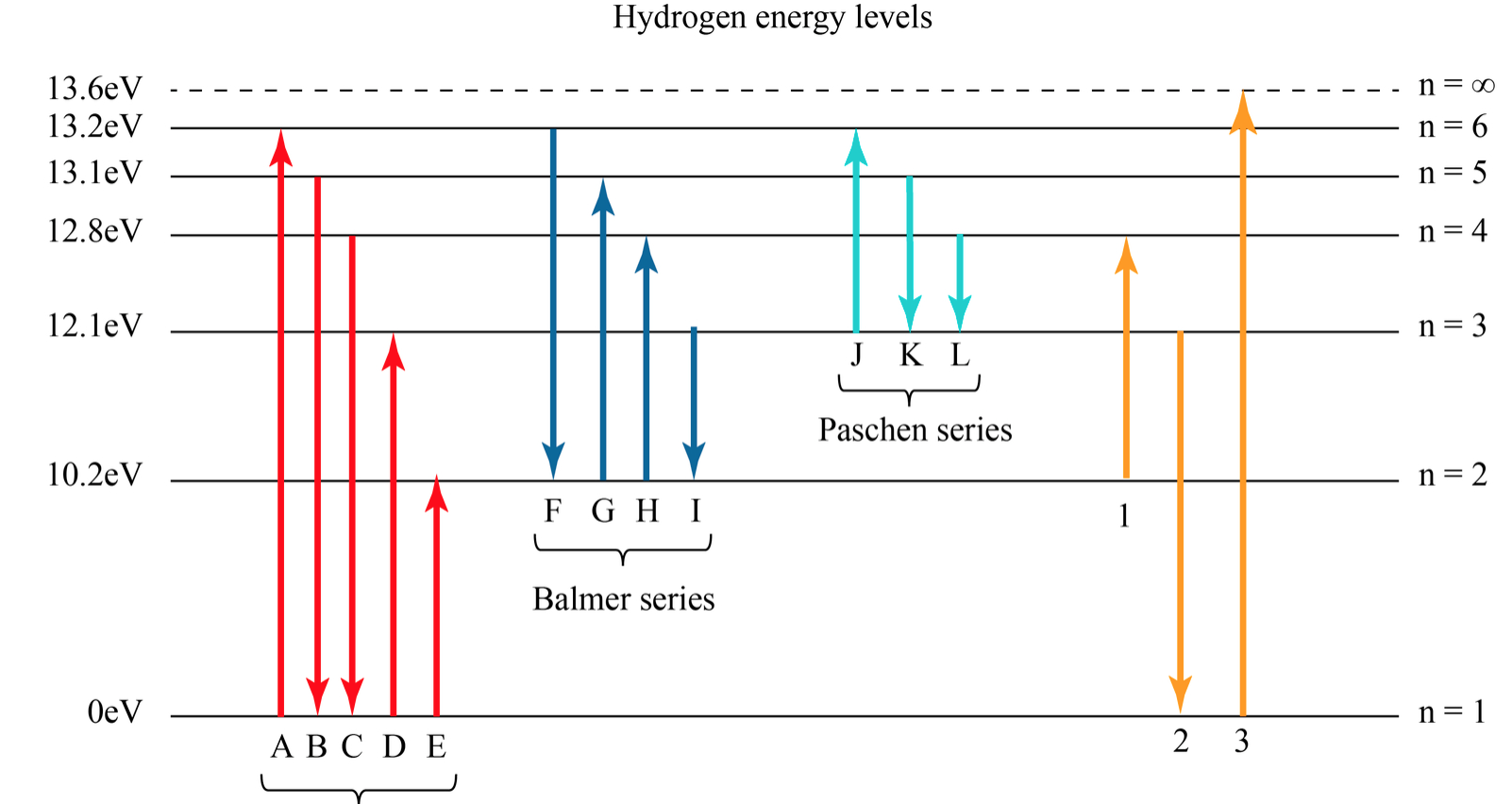
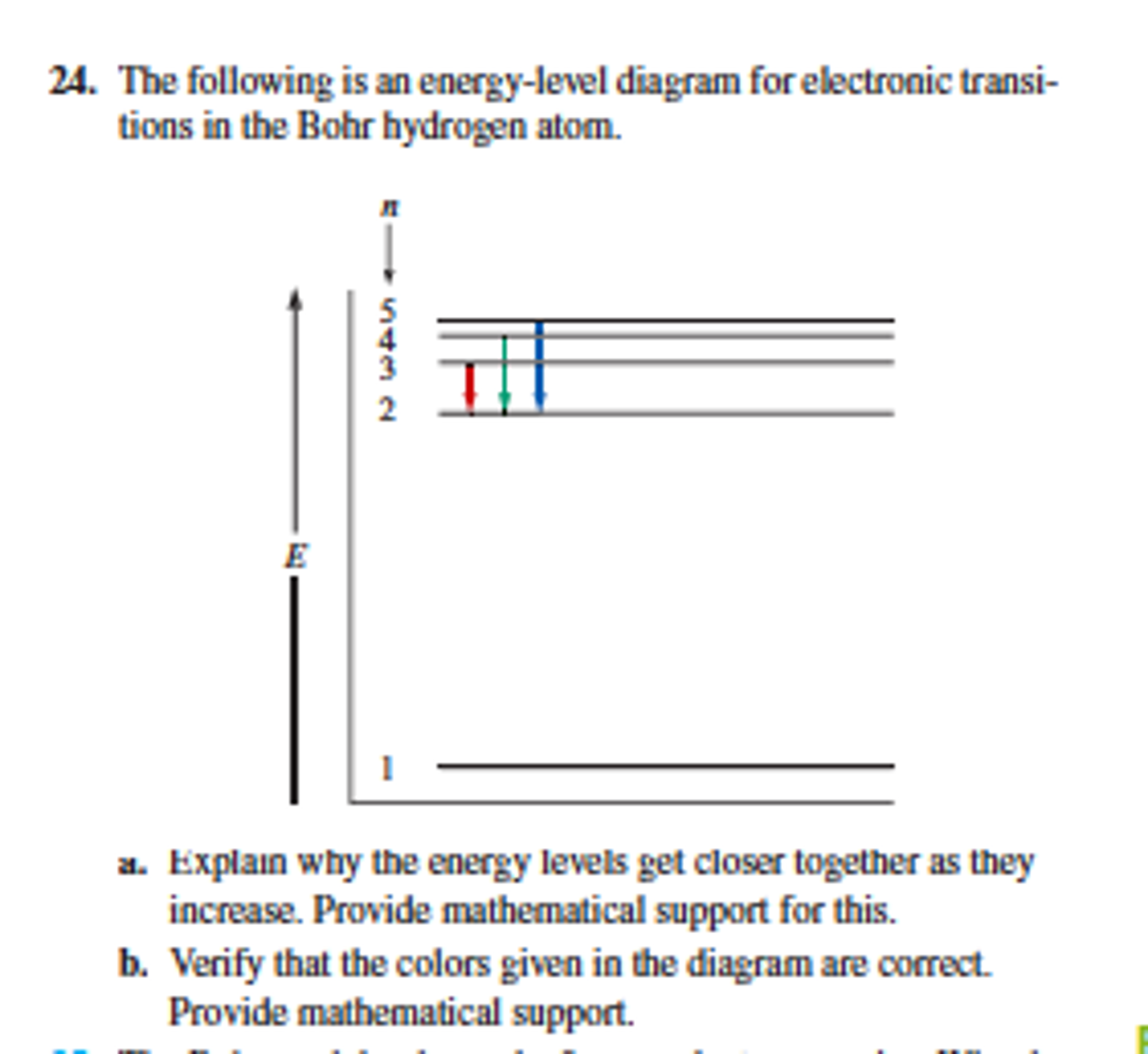
The energy for the first energy level is equal to negative 13.6. E two is equal to negative 3.4, and E three is equal to negative 1.51 electron volts. So energy is quantized using the Bohr models, you can't have a value of energy in between those energies.

The schematic energy level diagram for the levels (central panel)... Download Scientific Diagram
The energy level diagram gives us a way to show what energy the electron has without having to draw an atom with a bunch of circles all the time. Let's say our pretend atom has electron energy levels of zero eV, four eV, six eV, and seven eV. Note that moving left or right on an energy level diagram doesn't actually represent anything.

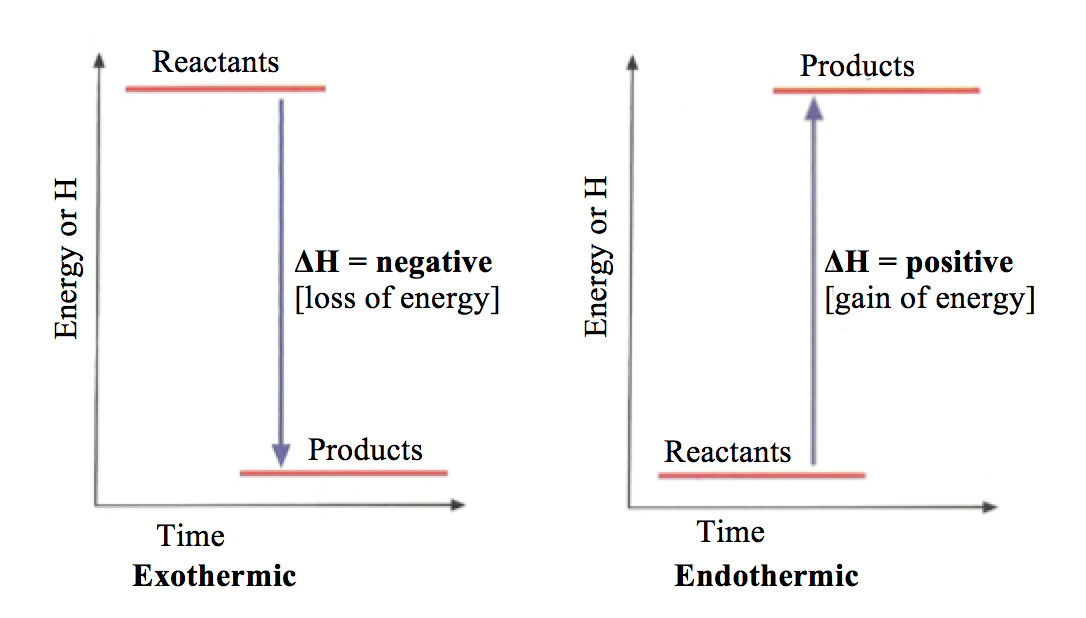
Energy level diagrams Endothermic & Exothermic reactions
energy level, in physics, any discrete value from a set of values of total energy for a subatomic particle confined by a force to a limited space or for a system of such particles, such as an atom or a nucleus. A particular hydrogen atom, for example, may exist in any of several configurations, each having a different energy.
PPT Energy Level Diagrams PowerPoint Presentation, free download ID2061569
Energy level diagrams are a means of analyzing the energies electrons can accept and release as they transition from one accepted orbital to another. These energies differences correspond to the wavelengths of light in the discreet spectral lines emitted by an atom as it goes through de-excitation or by the wavelengths absorbed in an absorption.
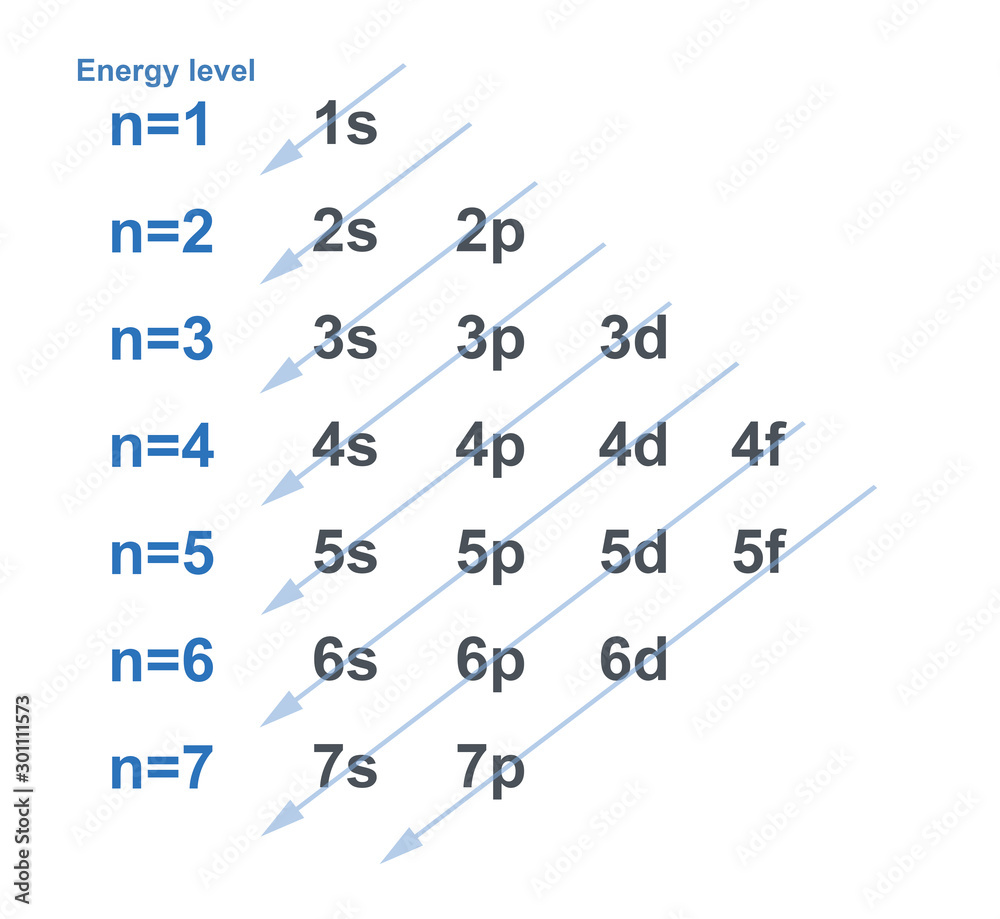
chart of electron configuration with each energy level for element in chemistry. Stock
Elements of Energy Diagrams. First of all, it should be noted that we will be confining ourselves to energy diagrams for 1-dimensional motion. This dimension will be represented by the horizontal axis, and the vertical axis will have units of energy. Secondly, the physical systems represented by energy diagrams will involve only one.
PPT Energy Level Diagrams PowerPoint Presentation, free download ID2061569
Introduction Glossary History Background Fundamentals Experiments Formulations Equations Interpretations Advanced topics Scientists v t e A quantum mechanical system or particle that is bound —that is, confined spatially—can only take on certain discrete values of energy, called energy levels.
Chem Energy Levels Scientific Tutor
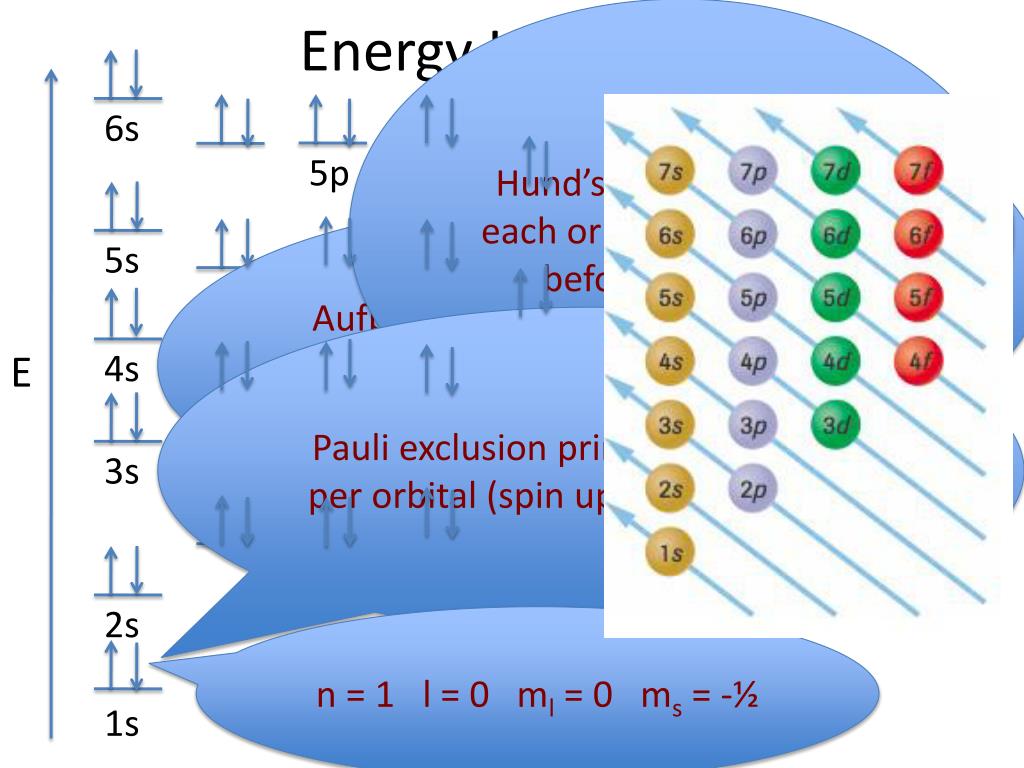
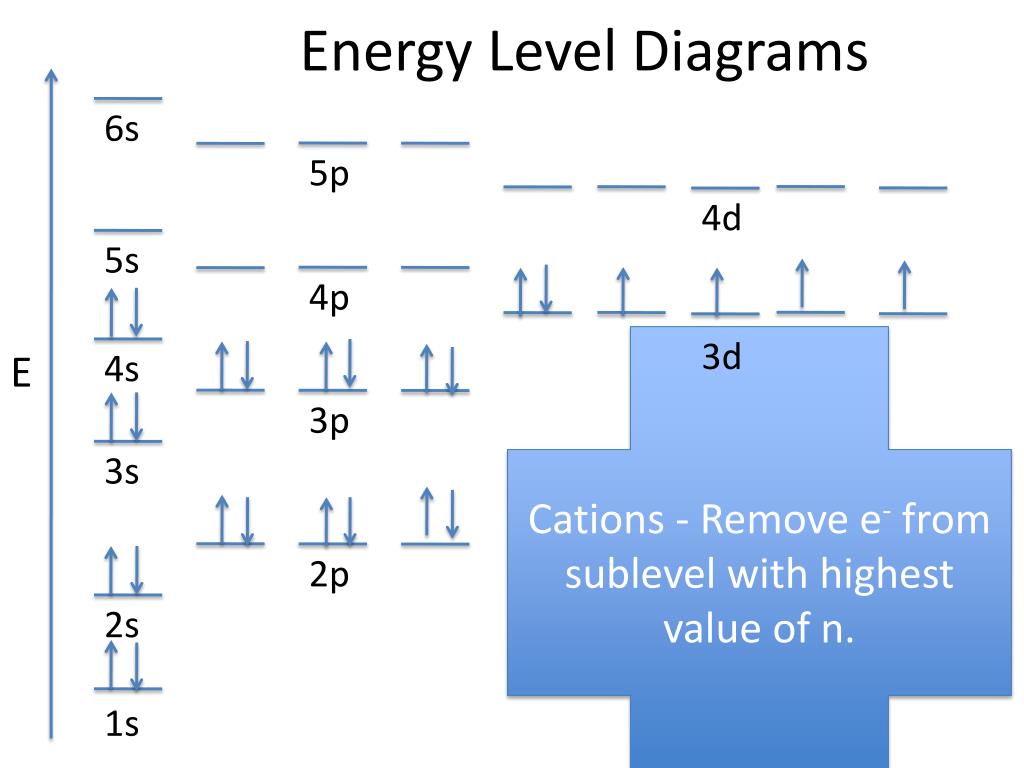
Figure 6.24 Generalized energy-level diagram for atomic orbitals in an atom with two or more electrons (not to scale). Electrons in successive atoms on the periodic table tend to fill low-energy orbitals first. Thus, many students find it confusing that, for example, the 5 p orbitals fill immediately after the 4 d, and immediately before the 6 s.

Schematic energylevel diagram of Tb 3+ and Eu 3+ in Ca 8 MgTb(PO 4 )... Download Scientific
Energy Level Diagrams for A=12 Available in the following years: ( 2017 ), ( 1990 ), ( 1985 ), ( 1980 ), ( 1975 ), ( 1968 ), ( 1959 ) A=12 Energy Level Diagrams from (2017KE05) PNG (Portable Network Graphics): 12 Be (45 KB) 12 B (156 KB) 12 C (449 KB) 13 B β - n decay scheme (49 KB) 13 O β + p decay scheme (67 KB) 12 N (286 KB) 12 O (15 KB)

Schematic energy level alignment of various CGLs. Energy level diagrams... Download Scientific
Key points Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. Bohr's model calculated the following energies for an electron in the shell, n : E ( n) = − 1 n 2 ⋅ 13.6 eV

physical chemistry LennardJones potential and vibrational energy level diagram explanation
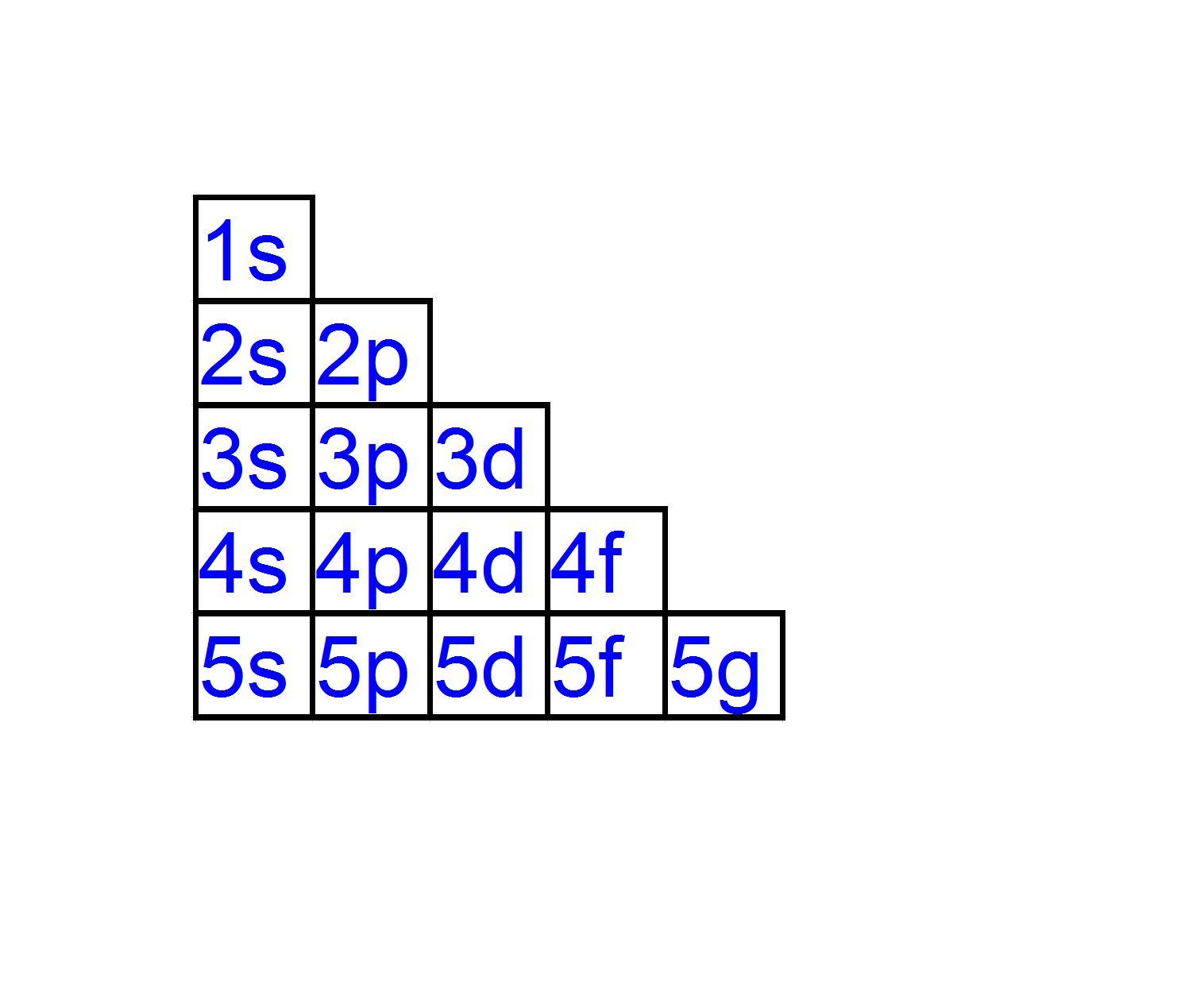
An energy level diagram is a graphical representation of the allowed energy state of a system. Only electronic states, vibrational states, or both electronic and vibrational states may be depicted in an energy level diagram. There are mainly 4 energy levels: s, p, d and f.
Solved The following is an energylevel diagram for
Energy Diagrams. Endothermic and exothermic reactions can be visually represented by energy-level diagrams like the ones in Figure \(\PageIndex{2}\). In endothermic reactions, the reactants have higher bond energy (stronger bonds) than the products.Strong bonds have lower potential energy than weak bonds.

Energy Level Definition, Equation (w/ Diagrams) Sciencing
Energy-Level Diagrams. Because electrons in the σ 1 s orbital interact simultaneously with both nuclei, they have a lower energy than electrons that interact with only one nucleus. This means that the σ 1 s molecular orbital has a lower energy than either of the hydrogen 1s atomic orbitals. Conversely, electrons in the \( \sigma _{1s}^{\star } \) orbital interact with only one hydrogen.

Energy level diagram of π 4 He + atom. On the left scale the... Download Scientific Diagram
Energy level diagrams are the representation of placements or arrangements of orbitals (also known as subshells) according to their increasing energy levels. Above is the blank energy level diagram which can be used to represent the electrons for any atom under study. Energy level diagrams are known as Grotrian diagrams.

Atomic Energy Level Diagram alternator
What is an energy level diagram? In chemistry, an electron shell, or energy level, may be imagined as an orbit with electrons around the nucleus of an atom. Bohr developed this model of the atom which says the electrons revolve around the nucleus in a circular path called an orbit.
